

EUROLAB, with its state-of-the-art accredited laboratories and expert team, provides precise and fast testing services within the scope of EN ISO 16061 testing. This standard specifies general requirements for instruments to be used with inactive surgical implants.
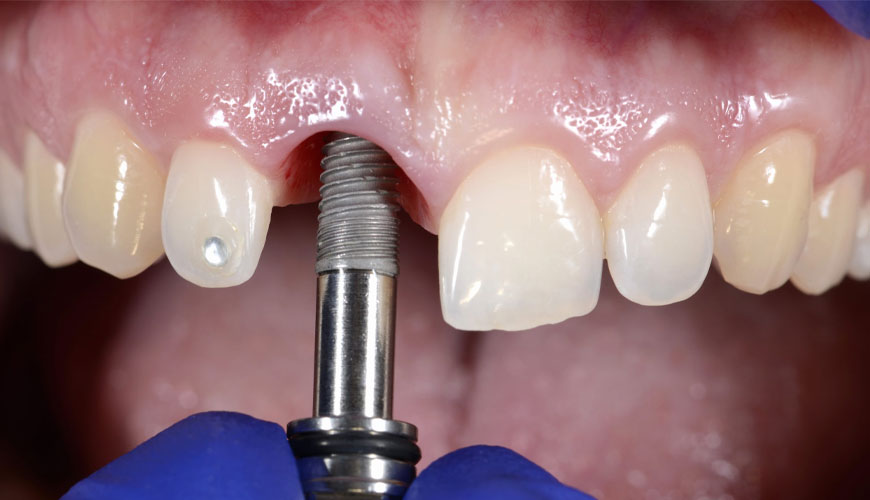
These requirements apply to tools when they are manufactured and when they are supplied after refurbishment.
In this document, unless otherwise specified, the term "instrument" refers to an instrument for use with inactive surgical implants.
This document also applies to devices that can be connected to powered systems, but not to the powered systems themselves.
Regarding safety, this document specifies requirements for intended performance, design features, materials, design evaluation, manufacturing, sterilization, packaging, and information provided by the instrument manufacturer, hereinafter referred to as the manufacturer.
This document does not apply to dental implants, transendodontic and transradicular implants and instruments associated with ophthalmic implants.
EUROLAB assists manufacturers with EN ISO 16061 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.