

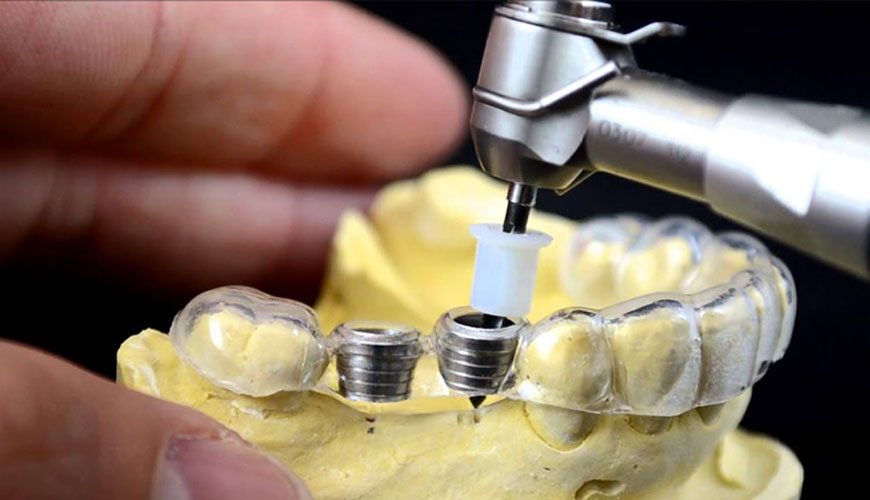
EUROLAB laboratory provides testing and compliance services within the scope of EN ISO 14630 standard. This standard specifies general requirements for inactive surgical implants. It does not apply to dental implants, dental restoration materials, transendodontic and transradicular implants, intraocular lenses and implants using live animal tissue.
Regarding safety, EN ISO 14630 specifies requirements for intended performance, design features, materials, design evaluation, manufacturing, sterilization, packaging, and manufacturer-provided information, and tests to demonstrate compliance with these requirements.
There are three levels of standards for inactive surgical implants and associated instrumentation. For the implants themselves, the general requirements for Inactive surgical implants, with level 1 being the highest, Special requirements for Inactive surgical implant families, Special requirements for Inactive surgical implant types.
Level 1 standards such as this International Standard and Reference contain requirements that apply to all inactive surgical implants.
Level 2 standards apply to a more limited set or family of inactive surgical implants, such as those designed for use in neurosurgery.
Level 3 standards apply to certain types of implants in the inactive surgical implant family, such as hip joints or arterial stents.
EN ISO 14630 Test Scope
This test method specifies general requirements for inactive surgical implants, hereinafter referred to as implants. This test is not applicable to dental implants, dental restoration materials, transendodontic and transradicular implants, intraocular lenses and implants using live animal tissue.
Regarding safety, this test method specifies requirements for intended performance, design features, materials, design evaluation, manufacturing, sterilization, packaging, and manufacturer-provided information, and tests to demonstrate compliance with these requirements.
Additional tests are given or referenced in level 2 and level 3 standards. This International Standard does not require the manufacturer to have a quality management system in place. However, the implementation of a quality management system may be appropriate to help ensure that the implant achieves its intended performance.
EN ISO 14630 testing services, which are meticulously carried out by our engineers, are provided by Eurolab to businesses within the scope of medical tests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.