

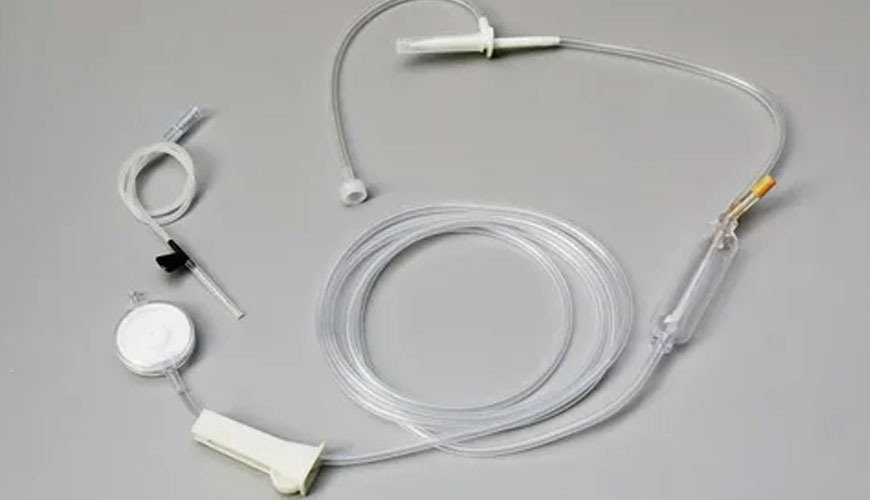
EUROLAB laboratory provides testing and compliance services within the scope of EN ISO 8536-15 standard. This standard specifies requirements for single-use infusion sets that use photoprotective agents in fluid pathway materials (hereinafter abbreviated as "photoprotective infusion sets").
This standard also provides guidelines for the performance and quality characteristics of materials used in light.
With the continuous development of infusion technology and ever-increasing clinical requirements, some infusion sets need to be adapted to specific clinical requirements.
Some pharmaceuticals, such as sodium nitroprusside, nitroglycerin, and vitamin B2, are photosensitive and must be clinically inoculated under photoprotective conditions; This standard applies to such sets.
This standard specifies light transmission requirements for the drip chamber and tube. As other components are limited by their external dimensions, they are not subject to light transmittance requirements and it is at the discretion of the manufacturer whether they will be light shielding.
EUROLAB assists manufacturers with EN ISO 8536-15 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.