

EUROLAB, with its state-of-the-art accredited laboratories and expert team, provides precise and fast testing services within the scope of EN ISO 8872 testing. This standard specifies general requirements and test methods for injection vials and aluminum caps for infusion and transfusion vials.
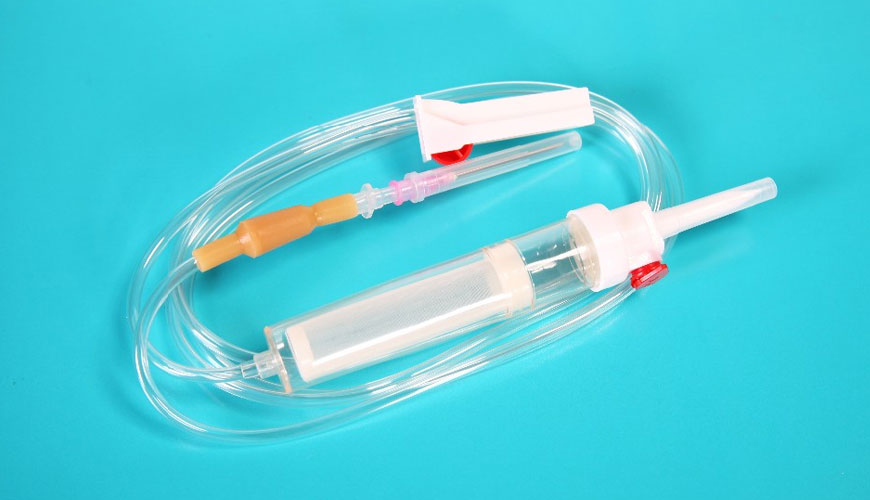
Transfusion, healthy people between the ages of 18-65 and body weight over 50 kg can donate blood. Men, most often once every 3 months, up to 4 times a year; women, on the other hand, can donate blood up to 4 times a year, most frequently once every 3 months.
Thanks to modern advances in infusion therapy, people with serious illnesses can be successfully treated with intravenous or subcutaneous methods.
We can summarize the main purpose of the injection unit, namely the injection group, as melting the plastic material and pressing the mold. For the production of parts with the same weight and the same quality, the amount of material pressed into the mold should be the same every time. For this, the injection group must be able to print homogeneous material at the same temperature.
EUROLAB assists manufacturers with EN ISO 8872 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.