

The ASTM F384 test and specification method provides a comprehensive reference for angled devices used in surgical internal fixation of the skeletal system. This standard establishes consistent methods for classifying and describing the geometric and performance characteristics of angled devices.
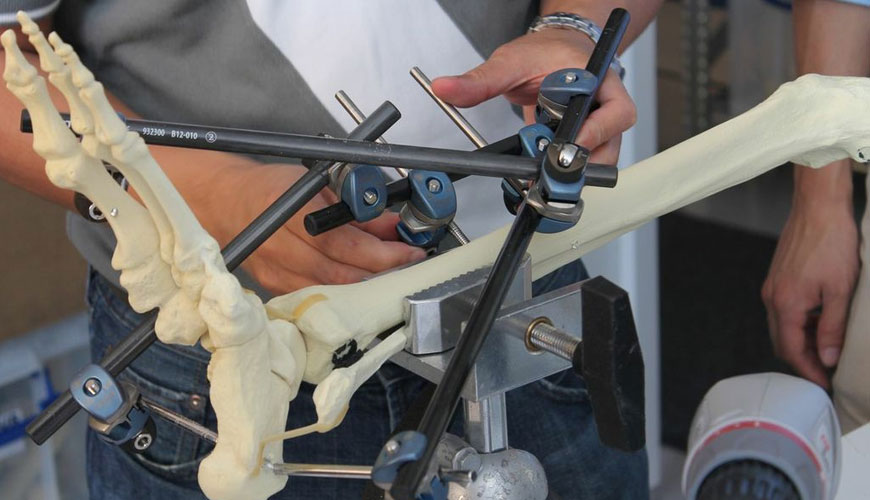
This standard also provides a catalog of standard specifications specifying material, labeling, and handling requirements, and standard test methods for measuring performance-related mechanical properties determined to be important to the in vivo performance of angled devices.
The test method creates a uniform cantilever bending fatigue test to characterize and compare the fatigue performance of different angled device designs. This test method can be used to determine the fatigue life of an angled device in a particular shape or under a range of maximum bending moment conditions. Also, this test method can alternatively be used to estimate the fatigue strength of an angled device for a given number of fatigue cycles.
The test method uses a simplified angled device cantilever bending load model that may not be fully representative of the in situ loading configuration. The user should note that the test results generated by this test method cannot be used to directly predict the in-vivo performance of the angled device under test. Data generated from this test method can be used to make relative comparisons of different angled device designs.
This test method may not be suitable for all types of implant applications. The user is cautioned to consider the suitability of the method, taking into account the tested devices and their potential applications.
This test method assumes that the angled device is manufactured from a material that exhibits linear elastic material behavior; therefore, this test method is not applicable for testing angle devices made of materials that exhibit nonlinear elastic behavior.
This test method is limited to testing angled devices in the linear elastic range of the material; therefore, this test method is not applicable to testing angled devices under conditions that will approach or exceed the bending strength of the angled device under test.
It is not the purpose of this standard to describe performance levels or case-specific clinical performance for angle devices because there is insufficient information to predict the consequences of their use in individual patients for certain activities of their daily life. In addition, this standard does not define or specify specific designs for angled devices used in surgical internal fixation of the skeletal system.
This standard may not be suitable for all types of angled devices. The user is cautioned to consider the conformity of this standard, taking into account the particular angled device and its possible application.
This standard includes the following test methods used to determine the following angle device mechanical performance characteristics:
Standard test method for single-cycle compression twist testing of metallic angle orthopedic fracture fixation devices,
Standard test method for determining the bending fatigue properties of metallic angle orthopedic fracture fixation devices.
Values stated in SI units should be accepted as standard. No other units of measurement are included in this standard.
Our organization also provides testing services within the scope of ASTM F384 Metallic Angle Orthopedic Fracture Fixation Devices within the scope of Standard Features and Test Methods within the framework of laboratory testing services.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.