

It is a common standard for orthopedic implant manufacturers to apply permanent identification to implant components. In this context, Application F86 describes recommended positions and marking methods for metallic implants.
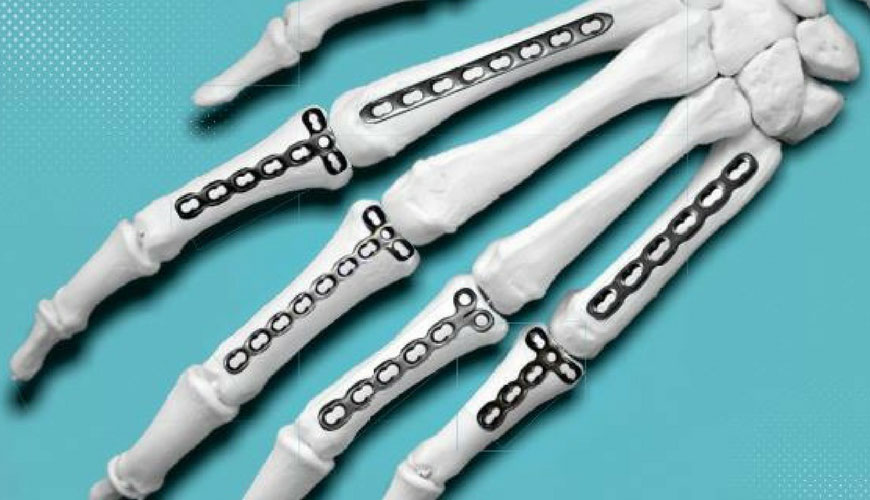
The purpose of this application is to recommend permanent marking of orthopedic implants and to recommend the amount of practical information that should be included in marking. However, in some cases marking is considered impractical.
This standard does not purport to address all, if any, safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and to determine the applicability of regulatory restrictions prior to use.
EUROLAB assists manufacturers with ASTM F983-86 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.