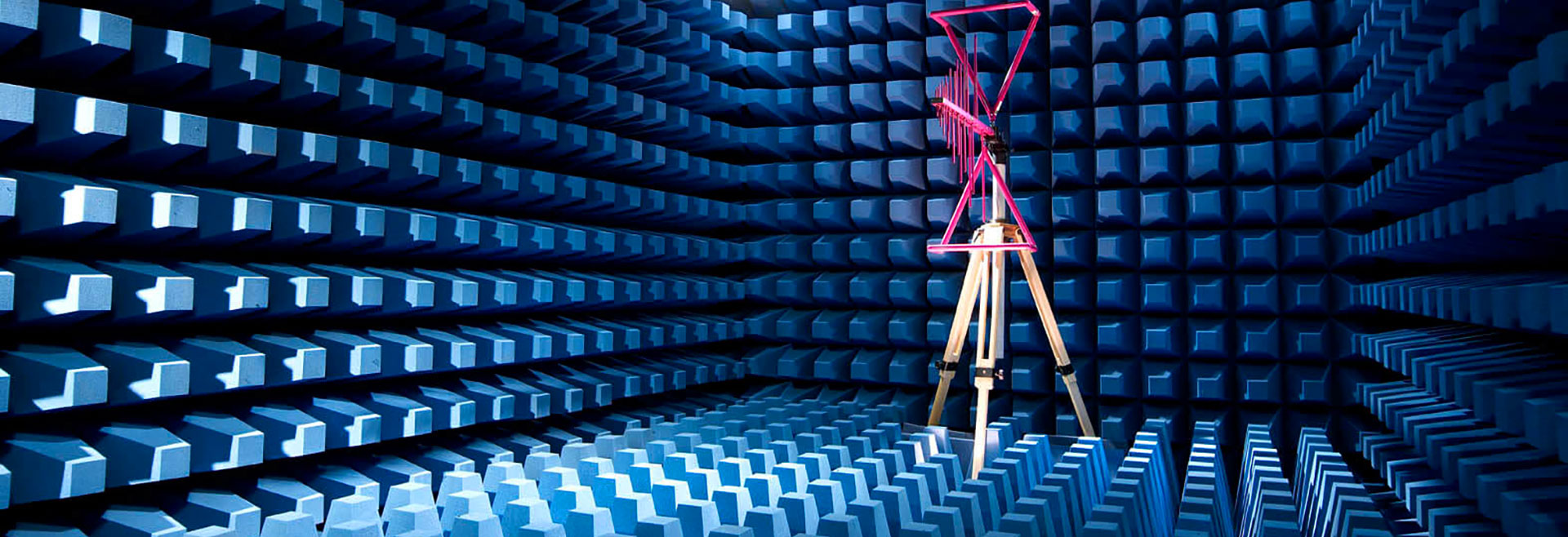

EUROLAB is proud to be the industry leader in the AIM 7351731 compliance test. EUROLAB is one of the first laboratories to offer customers tests according to these standards.

AIM 7351731 test It is now strictly mandatory by the FDA and is particularly applicable to medical electrical equipment and systems. AIM 7351731 sets out test methods / levels for (1) electromagnetic immunity of such equipment / systems and (2) radio frequency identification (RFID) readers included in such equipment / systems.
It is important to note that AIM 7351731 applies to all electrical medical devices, not just medical devices containing wireless devices / components. If you have questions about the effect of AIM 7351731 on your device, you can contact us.
AIM Standard does not consider other technologies such as Wi-Fi and ultra-broadband (UWB). It focuses on the most common RFID technologies currently used in healthcare environments.
AIM 7351731 guides RFID solutions, whether you're a designer, medical facility engineer, or administrator. AIM 7351731 can help you determine whether medical electrical devices / device or medical electrical system and RFID solution environment are compatible.
We can perform the tests required by AIM 7351731 and provide test reports to the FDA for your medical device approval.
AIM 7351731 - Electromagnetic Immunity Test for Exposure to Medical Electrical Devices and System RFID Readers is a consensus standard recognized by the FDA.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.