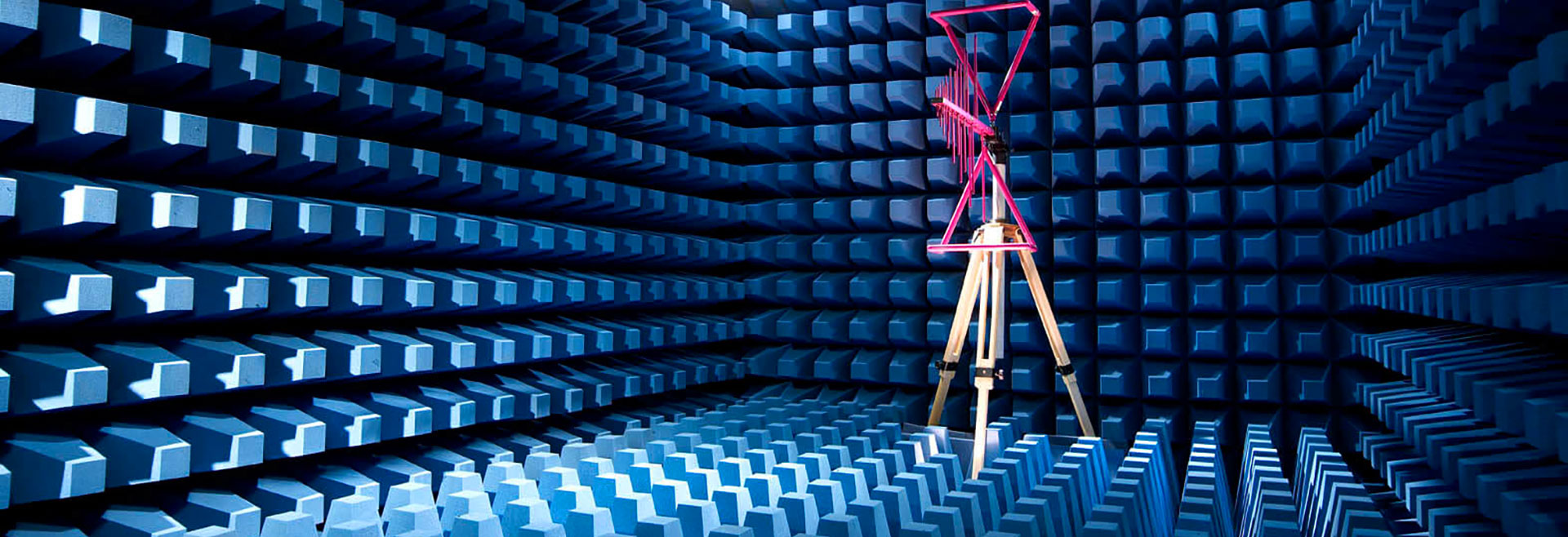

Below is a summary of the most important changes to this standard. As with other standard changes, failure to complete these new requirements on time can result in costly delays in placing your device on the market.
While version 60601 of IEC 1-2-3 is still in use, it is often difficult to determine which version of IEC 60601-1-2 should be used. EUROLAB can assist with your FDA medical test and medical device testing and certification.
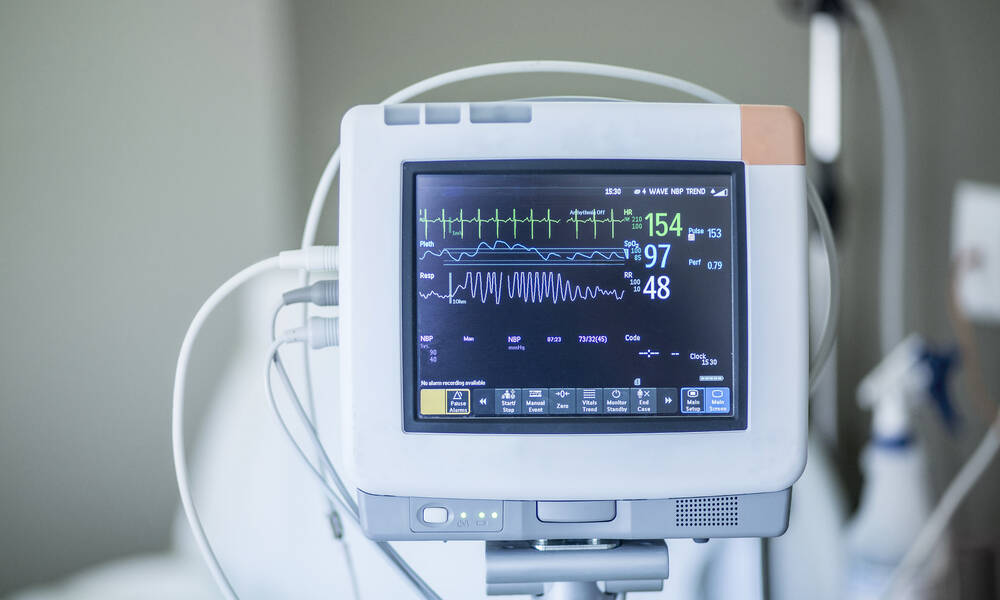
Your medical device must comply with version 31 of IEC 2018-60601-1 by 2 December 4 for Europe, the United States (FDA) and Canada.
However, while the U.S. FDA is submitting new applications, it now chooses products that will be considered for the 4th edition. This is especially true for medical devices used in the home care environment.
The FDA does not require compliance with 4th edition for older devices unless the product is modified.
In the European Union (accepting the CE Mark), medical devices must comply with the 60601th edition of EN 1-2-4. There is no allowance for older devices, as the FDA has allowed.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.