

EUROLAB laboratory provides testing and compliance service within the scope of USP 1113 standard. This standard specifies the identification of microorganisms.
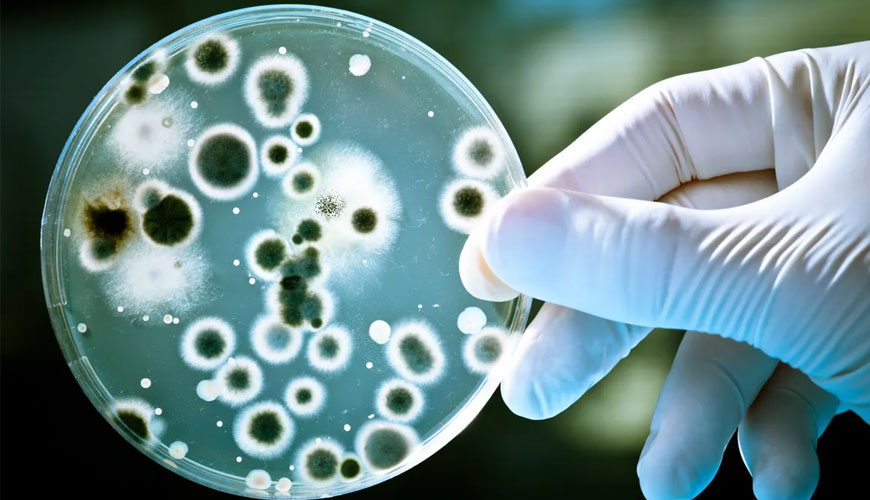
Identification of microorganisms is a key element in identifying the root cause of sterility test failure or identifying the source of any ongoing microbiological investigation. Various Guidelines, Regulations and Testing Standards recognize the importance of obtaining the identity of the microorganism.
Microbial identification is a key component of processes that exceed Alert or Action Levels, include “indicated” microorganisms, cause Out of Trend (OOT), create failures within Sterility Tests, and contribute to other product failures.
Monographs for non-sterile products refer to this test, eg USP <1111> Microbiological Properties of Non-Sterile Pharmaceutical Products. Additionally, USP <61> and <62> form the basis for many other USP General Division tests, which include bioburden, antimicrobial efficacy, environmental, and utilities testing.
EUROLAB assists manufacturers with USP 1113 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.