

In many cases, liquid-borne bacteria pass through a material barrier in the wet state, such as skin flora passing through a coating material. ISO 22610 specifies a test method, with associated test apparatus, for determining the resistance of a material to the penetration of bacteria carried by a liquid when subjected to mechanical friction.
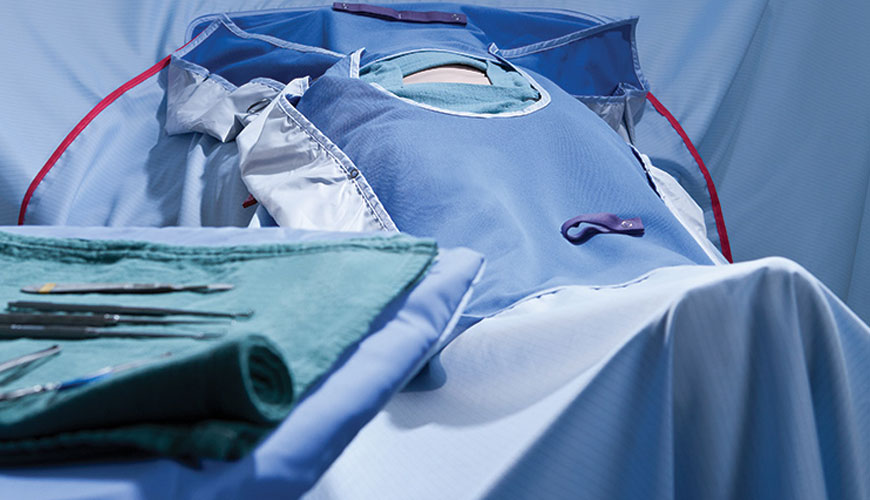
To perform the test, a sample of the material to be tested is placed on an agar plate. This material tested is 25 x 25 cm square pieces. A donor carrier plate contaminated with a Staphylococcus aureus culture is placed over the sample of material to be tested. This sheet is also covered with a high density polyethylene sheet.
The three plates are held together and stretched by steel rings. An eccentrically rotating piston moves over the three layers applying a moving force for 15 minutes to bring the sample of material under test into contact with a small area of the culture medium surface.
Due to the combined effect of friction and fluid migration, bacteria can migrate from the donor slide from the test material sample to the agar surface. The pressure applied in the test is intended to simulate the type of pressure exerted by the surgeon's elbow during a procedure and was developed specifically to measure bacterial penetration through disposable and reusable materials used during surgery.
5 replicates of each test material should be tested. For each of the fragments or replicates of the sample, this procedure is repeated with the same donor plate for 15 minutes with five plates of culture medium and likewise 5 consecutive tests to estimate penetration through air. At the end of the procedure, the donor layer is removed and the top of the test material sample is examined for contamination in another petri dish.
EUROLAB assists manufacturers with EN ISO 22610 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.