

ISO 10555-1 defines standards for intravascular sterile and disposable catheters. Types of catheters that can be tested according to this standard include general intravascular catheters, central venous catheters, balloon dilation catheters, and peripheral over-needle catheters.
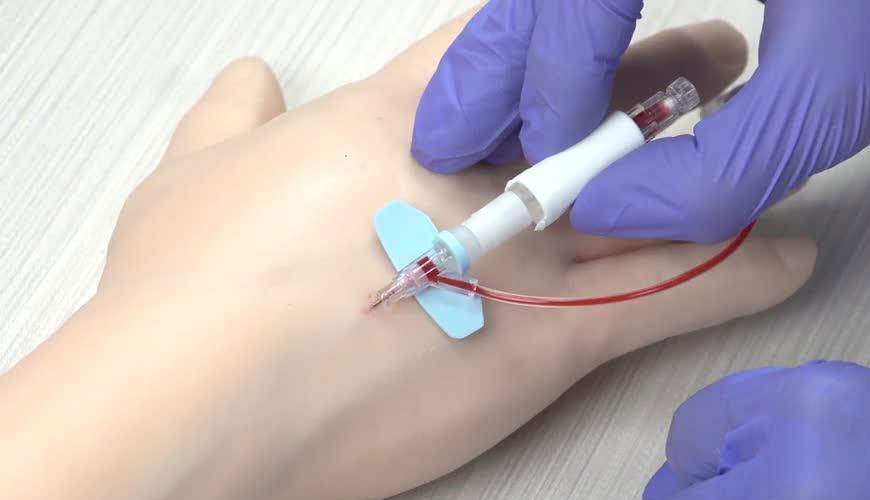
General catheter testing requirements;
Intravascular catheters are designed for tasks such as supporting the delivery of drugs and implantable devices into the human body. The best way to ensure that these devices are safe, maintain their quality and perform well is to subject them to a series of rigorous performance and durability tests.
EUROLAB assists manufacturers with ISO 10555-1 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.