

EUROLAB laboratory provides testing and compliance services within the scope of ISO 13544 standard. Developed by the International Standards Organization (ISO), the ISO 13544 standard specifies requirements for the safety, performance and testing of general purpose nebulization systems intended for the delivery of liquids to humans via the respiratory system in the form of a continuous or breath-actuated aerosol.
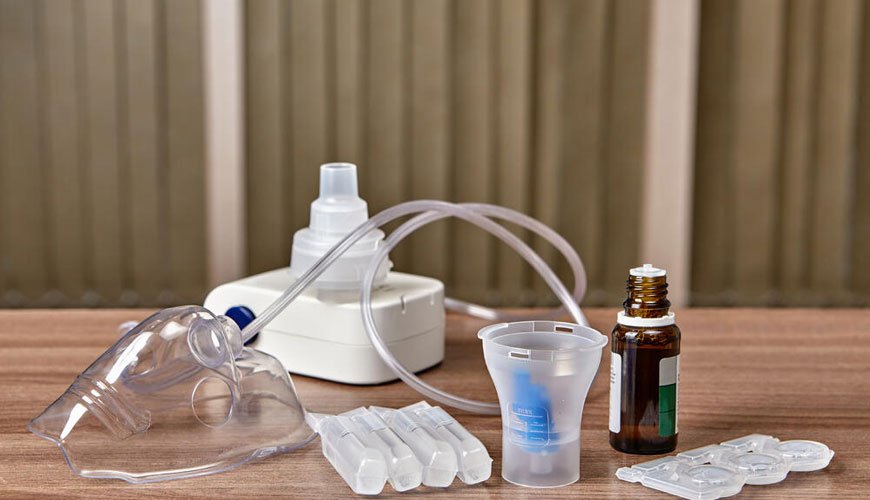
Nebulizers are widely used to deliver drugs in aerosol form to humans through the respiratory system. These drugs may be in the form of a solution, suspension, or emulsion. Aerosol inhalation is the preferred route of administration for some drugs. Some drugs are intended for the treatment of systemic diseases, and other drugs are intended to treat respiratory diseases.
To achieve the intended treatment, aerosol particles may need to be deposited in certain parts of the respiratory tract. Particles of different sizes tend to accumulate in different parts of the respiratory system; therefore the performance profile and intended use of the nebulizer should be defined by the manufacturer and stated in the accompanying documentation. Nebulizers are also used for diagnostic purposes using radioisotopes and for lung challenge tests and delivery of vaccines.
This International Standard is based on the European Standard EN 13544-1. This International Standard has been developed to cover "general purpose" nebulizers. It has been specifically written to ensure that the results of the various tests declared by the manufacturer are meaningful to nebulizer users and buyers.
The purposes of this International Standard are to provide:
This International Standard applies, for example, to compressors, pipeline systems, cylinders, etc. gas-powered nebulizers and electrically-operated nebulizers [eg. rotating disc, ultrasonic, vibrating mesh (active and passive) and capillary devices] or manually operated nebulizers.
This International Standard does not apply to devices for nasal deposition. This International Standard does not apply to devices for humidification or hydration by supplying water in aerosol form only. This International Standard does not apply to drug-specific nebulizers (for example, metered dose inhalers, metered liquid inhalers, dry powder inhalers and their components).
Among the services provided by our organization within the framework of material testing services, there are also ISO 13544 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.