

EUROLAB laboratory provides testing and compliance services within the scope of ISO 14708-1 standard. Developed by the International Standards Organization (ISO), this part of the ISO 14708 standard specifies generally applicable requirements for active implantable medical devices. The tests specified in ISO 14708 are type tests and will be performed on samples of an active implantable medical device to demonstrate conformity.
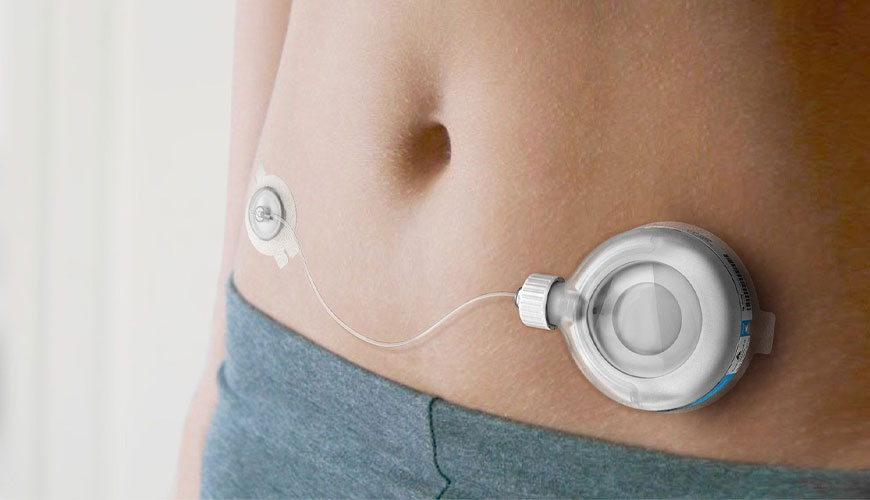
ISO 14708-1 is applicable not only to electrically powered active implantable medical devices, but also to devices powered by other energy sources (e.g. gas pressure or springs). ISO 14708-1 is also applicable to certain non-implantable parts and accessories of active implantable medical devices.
For certain types of active implantable medical devices, these general requirements are supplemented or replaced by the requirements of certain parts of ISO 14708. The device, commonly referred to as an active implantable medical device, may be a single device, a combination of devices, or a device or combination of devices and one or more accessories. It is not necessary for all of these parts to be partially or fully implantable, but some requirements for non-implantable parts and accessories need to be specified if they may affect the safety or performance of the implantable device.
This part of ISO 14708 specifies general requirements for active implantable medical devices to provide basic assurance of safety for both patients and users.
To minimize the potential for abuse of a device, this section of ISO 14708 also describes the comprehensive requirements for markings and other information to be provided as part of documentation relating to any active implantable medical device.
The general requirements for certain types of active implantable medical devices may be supplemented or replaced by the requirements of other parts of ISO 14708. The requirement of a particular part of ISO 14708 takes precedence over the corresponding requirement of this general part of ISO 14708.
Where specific parts of ISO 14708 are available, this general part of ISO 14708 is not intended to be used alone. Special care should be taken when applying this general part of ISO 14708 alone to active implantable medical devices for which a specific International Standard has not yet been published.
Among the services provided by our organization within the framework of material testing services, there are also ISO 14708-1 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.