

EUROLAB laboratory provides testing and compliance services within the scope of ISO 18562-4 standard. Developed by the International Organization for Standardization (ISO), this part of the ISO 18562 standard specifies tests for substances leached by liquid water by condensation into the gas tracts of a medical device, its parts or accessories, and intended to supply a substance to a patient through respiratory care or the respiratory tract in all environments.
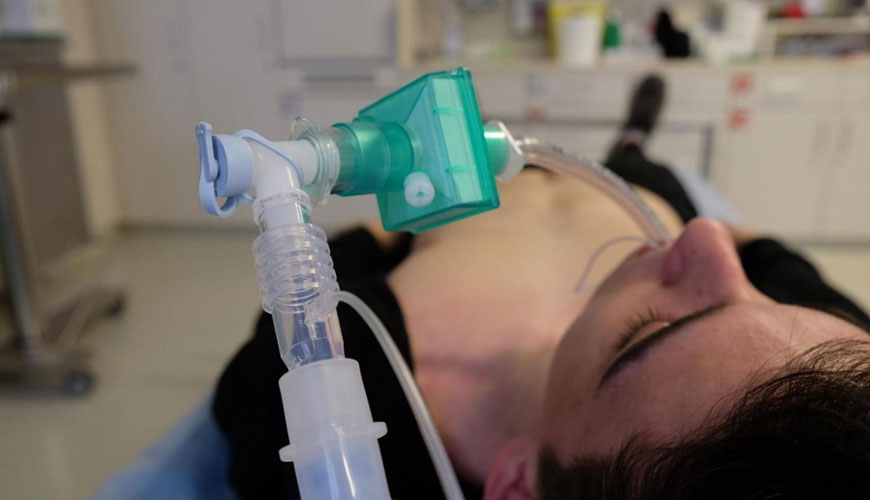
The tests of this document aim to determine the amount of water-soluble hazardous substances that leak from the medical device, its parts or accessories by condensation and are then carried to the patient by this fluid. This document sets the acceptance criteria for these tests.
ISO 18562-4 addresses possible contamination of the gas flow from the gas pathways that are then delivered to the patient. ISO 18562-4 applies to the expected service life of the medical device in normal use and takes into account the effects of any intended treatment or reprocessing.
ISO 18562-4 does not address the biological evaluation of the surfaces of gas tracts in direct patient contact. Requirements for direct contact surfaces are found in the ISO 10993 series.
Medical devices, parts, or accessories that contain gas paths discussed in this document include, but are not limited to, ventilators, anesthesia workstations (including gas mixers), breathing systems, oxygen protective devices, oxygen concentrators, nebulizers, low pressure hose assemblies. , humidifiers, heat and humidity exchangers, respiratory gas monitors, respiratory monitors, masks, mouthpieces, resuscitators, breathing tubes, respiratory system filters, Y-pieces and any respiratory accessories intended for use with such devices. The closed chamber of an incubator including the mattress and the inner surface of an oxygen hood are considered gas paths and are also covered in this document.
ISO 18562-4 does not address contamination already present in gas supplied from gas sources when medical devices are in normal use. This document is intended to be read in conjunction with ISO 18562‑1.
Contamination to a medical device from gas sources such as medical gas pipeline systems (including check valves at pipeline outlets), pressure regulator outlets connected to or integrated into a medical gas cylinder, or room air taken into the medical device is not addressed by the ISO 18562 series.
ISO 18562-4 does not address contact with drugs or anesthetics. If a medical device is intended for use with anesthetics or drugs, additional testing may be required.
This document aims to protect patients connected to medical devices from excessive amounts of harmful substances that may be present in condensed water in the gas paths of these medical devices. This document represents the application of best-known science in addressing the risks from potentially hazardous substances in gaseous condensate. The condensation itself will be distilled water condensed from the vapor phase, but liquid water in the respiratory system can filter or absorb other substances inside the medical device. This contamination may originate from the original manufacturing process or be produced by the medical device itself during use.
Among the services provided by our organization within the framework of material testing services, there are also ISO 18562-4 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.