

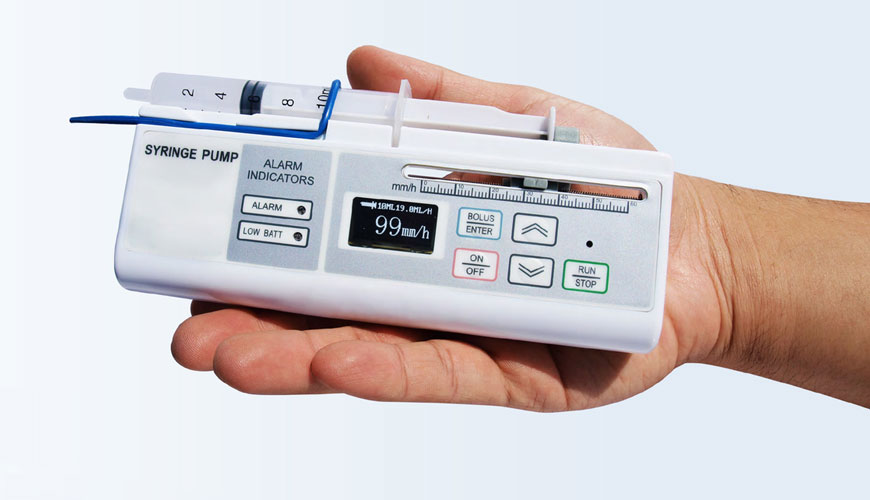
EUROLAB laboratory provides testing and compliance services within the scope of ISO 7886-2 standard. Developed by the International Organization for Standardization (ISO), this part of the ISO 7886 standard specifies requirements for sterile disposable hypodermic syringes with a nominal capacity of 1 ml and above, made of plastic materials and designed for use with power-driven syringe pumps.
This document does not apply to syringes with auto-deactivation of syringe features, syringes for use with insulin, disposable glass syringes, syringes prefilled with injection by the manufacturer, and syringes supplied with injection as kits to be filled by a pharmacist. It does not address compatibility with injection fluids.
It was recognized at an early stage in the preparation of this document that the absolute performance criterion was achieved with the combination of the powered syringe pump and the syringe operating as a complete system. The dependence of one element of the system on the performance of another is a key factor.
To ensure satisfactory operation of the system, it is essential that the manufacturer of one of these components liaises with the manufacturer of the other when evaluating changes to the design. In particular, a syringe manufacturer should provide information on performance characteristics and variation that can be expected, such as the relationships and tolerances between syringe sizes specified in this document and the force to move the plunger.
The choice of test rates for flow rate accuracy recognized that lower velocities are a worse case and cause large differences; however, due to the limitations of gravimetric test method error (due to factors such as balance stabilization and difficulty in measuring micro quantities of liquid using balances designed for static measurements), it was considered inappropriate to select rates less than 1 ml/h.
It is recognized that the start time and passing from park can affect pump forces and should be taken into account for exclusion if necessary. Syringe driver and measuring equipment specifications may affect test method error; therefore, it is recommended to include the appropriate level of accuracy and precision of the equipment and test method validations.
The use of syringes, originally designed and used as manually operated devices in syringe pumps, now makes it desirable to achieve much tighter tolerances on syringe sizes than is normally required for manual use.
It is understood that the degree of worldwide investment in molding and manufacturing equipment by all syringe manufacturers is largely beyond the reach of the syringe industry, with a change such as changing the diameters of push buttons or the inside diameter (ID) of the barrel.
Typically, the rigid height of a syringe has never been considered a particularly critical dimension. Their tolerances are relatively loose. The rigid height dimension is a function of not only the overall length of the piston rod and barrel, but also the thickness of the piston and barrel flanges.
Thanks to the relatively uncomplicated manufacturing process, the piston thickness can vary considerably. As all these components are produced in multi-cavity molds from many molds around the world, the cumulative build-up of over-tolerance from cavity-to-cavity and mold-to-die and position-to-position ensures that these previously non-critical dimensions are not instantly tightened.
Among the services provided by our organization within the framework of material testing services, there are also ISO 7886-2 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.