

EUROLAB laboratory provides testing and compliance services within the scope of ISO 80369-7 standard. Developed by the International Organization for Standardization (ISO), this part of the ISO 80369 standard specifies dimensions and requirements for the design and functional performance of small bore connectors intended for use for connections in intravascular applications or subcutaneous connections in subcutaneous applications of medical devices and accessories.
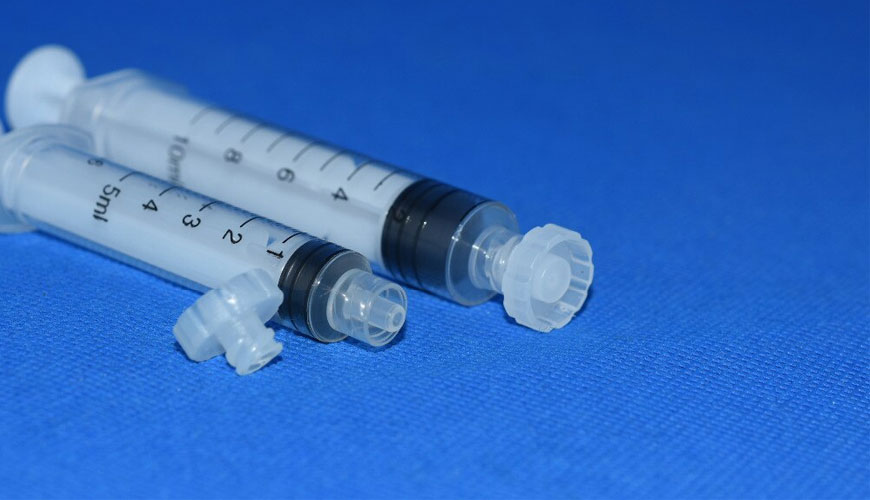
For example, hypodermic syringes and needles or intravascular (IV) cannulas with male and female Luer slip connectors and Luer lock connectors can be tested.
The Luer connector was originally designed for use at pressures up to 300 kPa. This standard does not specify requirements for medical devices or accessories that use these connectors. Such requirements are provided in specific documents for specific medical devices or accessories.
This document does not specify requirements for the following small diameter connectors specified in other documents:
Manufacturers are encouraged to include the small diameter connectors specified in this document in medical devices or accessories, even if they are not currently required by the relevant medical device documentation. Requirements for small diameter connectors as specified in ISO 80369 are expected to be included when the relevant specific medical device documentation is revised.
This standard was developed due to several events with disastrous consequences, caused by inappropriate medication, liquid nutritional formula, or intravenously administered air. Many events have been reported that led to the international recognition of the importance of these issues, and the need to develop special connectors for medical devices and accessories used to deliver fluids in other applications has been identified.
This document specifies the design, dimensions, and drawings of small bore connectors intended for use as conical fittings with a 6% (Luer) taper for connections in intravascular or hypodermic applications. Other parts of ISO 80369 contain requirements for small diameter connectors used in different application categories.
Connectors manufactured to the dimensions specified in this document are dimensionally incompatible with any of the other connectors for applications defined in the ISO 80369 document series for small diameter connectors. If attached to associated medical devices and accessories, these connectors should reduce the risk of delivery of air, non-vascular drug and nutrient formula via an alternative route such as intravenous or an airway device.
Among the services provided by our organization within the framework of material testing services, there are also ISO 80369-7 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.