

EUROLAB laboratory provides testing and compliance services within the scope of ISO 80601-2-61 standard. Developed by the International Standards Organization (ISO), this part of the ISO 80601 standard applies to the essential safety and essential performance of pulse oximetry equipment intended for human use. This includes all parts necessary for normal use, including the pulse oximetry monitor, pulse oximetry probe, and probe cable extender.
These requirements also apply to pulse oximetry equipment, including reworked pulse oximetry monitors, pulse oximetry probes, and probe cable extenders.
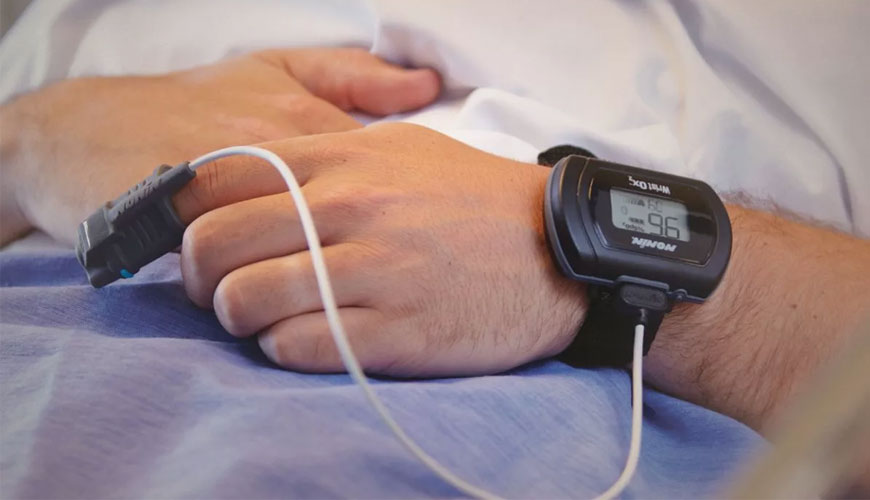
Estimation of arterial hemoglobin saturation and pulse rate using pulse oximetry is a common practice in many fields of medicine. This document covers basic safety and essential performance requirements achievable within the limits of current technology.
Intended uses of pulse oximetry equipment include, but are not limited to, estimation of arterial oxygen hemoglobin saturation and pulse rate of patients in professional healthcare settings, as well as patients in home healthcare settings and emergency medical services settings.
ISO 80601-2-61 does not apply to pulse oximetry equipment intended for use in laboratory research applications or oximeters that require a blood sample from the patient.
If an article or sub-article is specifically intended to apply only to my equipment or only to my systems, the title and content of that article or sub-article will say so. If this is not the case, the item or sub-clause applies to both my equipment and my systems, as relevant.
Hazards inherent in the intended physiological function of me equipment or me systems covered by this document are not covered by the specific requirements in this document, apart from the general standard.
ISO 80601-2-61 is also applicable to equipment and accessories used for the compensation or alleviation of disease, injury or disability. ISO 80601-2-61 does not apply to pulse oximetry equipment for fetal use only. ISO 80601-2-61 does not apply to remote or slave (secondary) equipment that displays Sp O2 values outside the patient environment.
Me equipment, which provides a choice between diagnostic and monitoring functions, is expected to meet the requirements of the appropriate document when configured for this function.
ISO 80601-2-61 is applicable to pulse oximetry equipment intended for use in extreme or uncontrolled environmental conditions outside the hospital environment or doctor's office, such as ambulances and air transport. Additional standards may apply pulse oximetry equipment for these use environments.
Among the services provided by our organization within the framework of material testing services, there are also ISO 80601-2-61 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.