

EUROLAB laboratory provides testing and compliance service within the scope of EN IEC 60601-2-16 standard. EN IEC 60601-2-16 applies to the essential safety and essential performance of hemodialysis, hemodiafiltration and hemofiltration equipment.
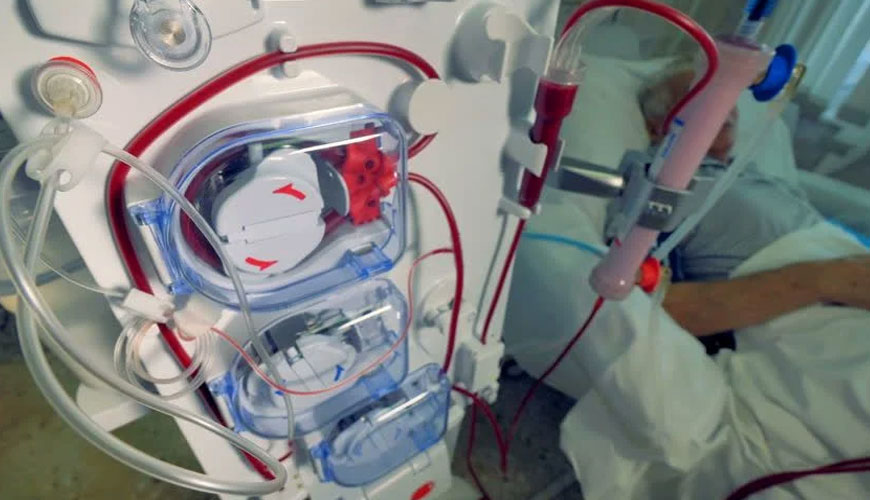
EN IEC 60601-2-16 does not consider the dialysis fluid control system and central distribution systems of hemodialysis equipment using dialysis fluid regeneration. However, it takes into account the specific safety requirements of such hemodialysis equipment regarding electrical safety and patient safety.
EN IEC 60601-2-16 specifies minimum safety requirements for hemodialysis equipment. These devices are intended for either use by medical personnel or by patients or other trained personnel under the supervision of medical expertise.
EN IEC 60601-2-16 includes all electromedical equipment intended to provide hemodialysis, hemodiafiltration and hemofiltration therapy to a patient with renal failure.
EUROLAB assists manufacturers with EN IEC 60601-2-16 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.