

EUROLAB laboratory provides testing and compliance services within the scope of EN ISO 8536-9 standard. EN ISO 8536-9 applies to single-use sterilized liquid lines for use with pressure infusion equipment up to a maximum of 200 kPa (2 bar).
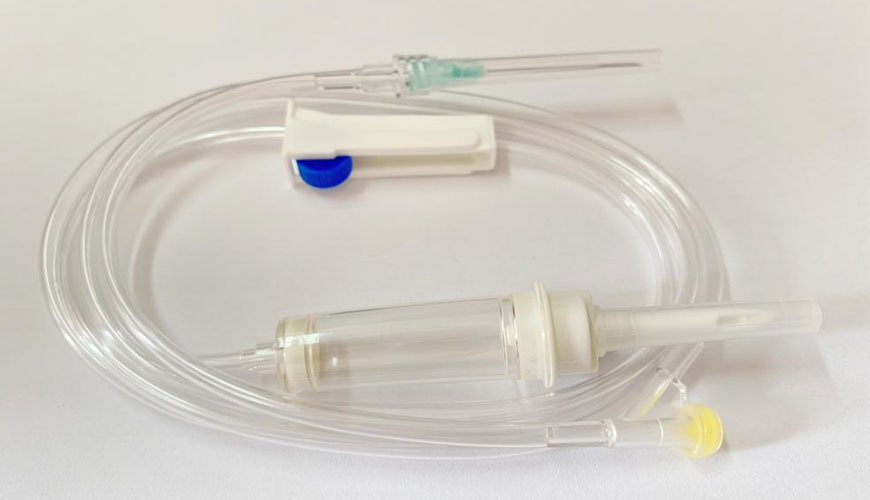
The following items are covered by EN ISO 8536-9:
In some countries, national pharmacopoeia or other national regulations are legally binding and take precedence over this part of EN ISO 8536.
Fluid lines should be evaluated for pyrogen-freeness using an appropriate test and results should demonstrate fluid lines are pyrogen-free. Guidance on pyrogenicity testing is provided in ISO 8536-4.
The fluid lines should be evaluated for free of hemolytic components and the result should show that the fluid lines are free from hemolytic reactions.
EUROLAB assists manufacturers with EN ISO 8536-9 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.