

IEC 60601-1 is a set of technical standards for the safety and effectiveness of medical electrical equipment. The IEC 60601-1 standard has a significant impact on the product development process, going beyond performance testing and verification. This is because product complexity often provides numerous potential test cases, permutations, and combinations in both normal and abnormal operating modes, and these cannot be evaluated only in the final design.
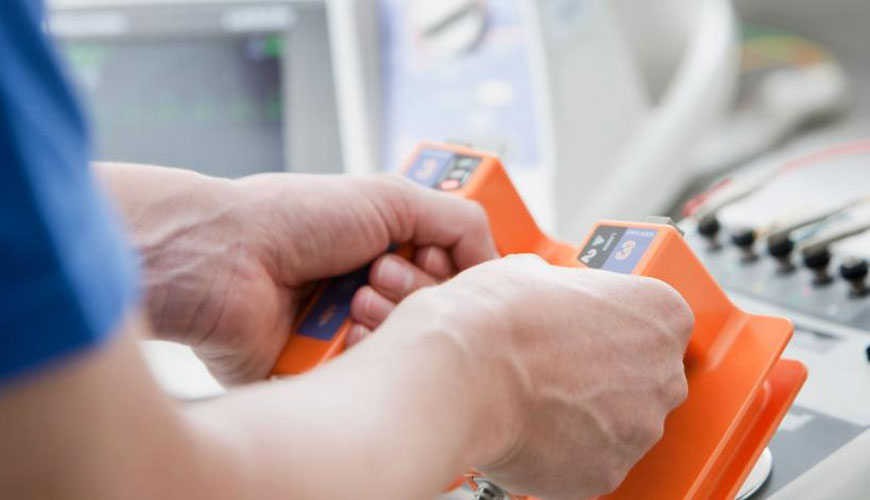
IEC 60601-1 is a set of technical standards that ensure the safety of medical electrical equipment. IEC 60601-1 deals with the essential safety and essential performance requirements of medical electrical equipment and serves to ensure that no electrical, mechanical, thermal or functional failure presents an unacceptable risk to patients or operators. It is assumed that public health authorities in many countries recognize IEC 60601-1-1 as a prerequisite for the commercialization of electrical medical equipment.
IEC 60601-1 is the newest general standard published with approximately 1500 single specific requirements. Requirements are generally considered cutting-edge and must be met in different markets around the world.
EUROLAB assists manufacturers with IEC 60601-1 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.