

EUROLAB laboratory provides testing and compliance services within the scope of ISO 80601-2-69 standard. Developed by the International Organization for Standardization (ISO), this part of the ISO 80601 standard applies to the basic safety and basic performance of a clinical thermometer with its accessories, hereinafter referred to as me equipment.
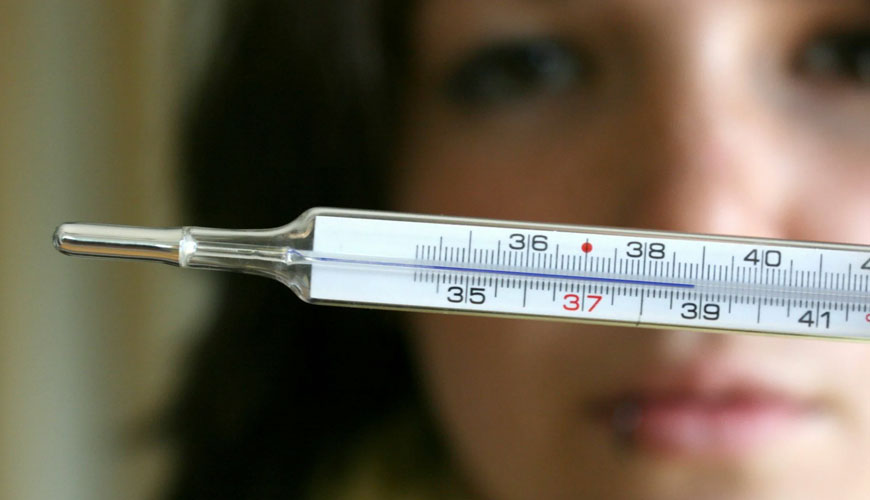
This document specifies the general and technical requirements for electrical clinical thermometers. This document applies to all electrical clinical thermometers used to measure the body temperature of patients.
Clinical thermometers can be equipped with interfaces to house secondary displays, printing equipment, and other ancillary equipment to create me systems. This document does not apply to auxiliary equipment. Me equipment that measures body temperature is covered by this document.
This document relates to electrical clinical thermometers that are currently available or will be available in the future. The purpose of the clinical thermometer is to evaluate the actual temperature of the reference body region. The patient's body temperature is an important vital sign in assessing general health, typically along with blood pressure and pulse rate. Determining whether a patient is febrile, febrile, or hypothermic is an important purpose of clinical thermometer because being feverish indicates that the patient is ill.
For a clinical thermometer operating in tuned mode, laboratory verification alone is not sufficient because the setup algorithm for deriving the outlet temperature includes characteristics of the patient and environment. Therefore, the clinical accuracy of a clinical thermometer operating in the tuned mode should be clinically verified using statistical methods comparing the outlet temperature with a reference clinical thermometer that has a certain clinical accuracy in representing a given reference body region temperature.
For a clinical thermometer operating in tuned mode, laboratory accuracy is verified in direct mode, and clinical accuracy is verified in tuned mode (study mode) with a sufficiently large group of human subjects.
ISO 80601-2-56 does not specify the requirements given in IEC 80601‑2‑59 for scanning thermographs intended to be used for individual non-invasive human febrile temperature screening of individual groups of people under indoor conditions.
If an article or sub-article is specifically intended to apply only to my equipment or only to my systems, the title and content of that article or sub-article will say so. If this is not the case, the item or sub-clause applies to both my equipment and my systems, as relevant.
Hazards arising from the intended physiological function of ME equipment or me systems covered in this document, IEC 60601-1, are not covered by the special requirements in this document.
Among the services provided by our organization within the framework of material testing services, there are also ISO 80601-2-56 standard tests. Do not hesitate to contact our laboratory EUROLAB for your testing and certification requests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.