

The IEC 60601-2-10 standard, prepared by the International Electrotechnical Commission (IEC), an organization affiliated to the International Standards Organization (ISO), describes the criteria for the basic safety and basic performance of nerve and muscle stimulators for use in physical medicine practice.
This standard has been published in our country by the Turkish Standards Institute with the following title: TS EN 60601-2-10 Electrical medical equipment - Part 2-10: Specific features for the basic safety and required performance of nerve and muscle stimulants.
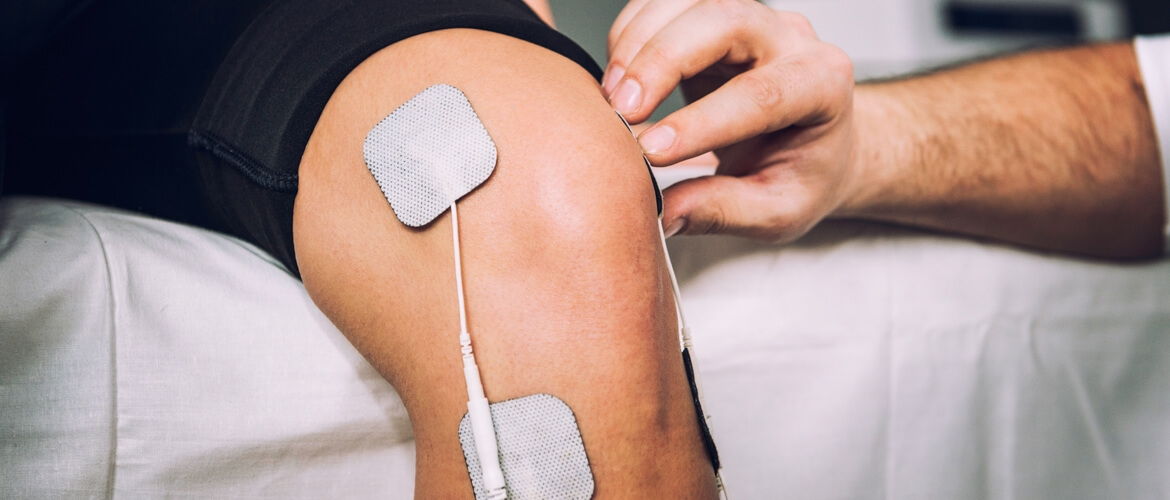
Included within the scope of said dtandard are transcutaneous (transcutaneous) electrical nerve stimulators and electrical muscle stimulators.
Although the use of electrical nerve stimulators to locate peripheral nerves has declined with the widespread use of ultrasonography techniques, it is still widely used today. The operation of these systems is based on stimulating nerves with electrical current at a certain threshold value and observing appropriate muscle contractions. Peripheral nerves are the nerves that connect the central nervous system and the external environment.
In short, nerve and muscle stimulating medical devices have been used for years for electrical stimulation of living tissues and for the diagnosis and treatment of nerve and muscle disorders. These devices are used for purposes such as increasing nerve and muscle strength, recovery of decreased nerve and muscle strength, tightening of muscles and regulation of blood circulation.
IEC 60601 1 10 A few standards that have been referred to and indispensable during the application of the standard are: IEC 60601-1-2 (general requirements for medical electrical equipment, basic safety and basic performance, assurance standard: electromagnetic compatibility, requirements and tests) and IEC 60601-1 (medical General requirements for electrical equipment, basic safety and basic performance).
Tests in accordance with IEC 60601-2-10 standard are also carried out by our organization within the scope of medical device tests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.