

The IEC 60601-2-27 standard, prepared by the International Electrotechnical Commission (IEC), an affiliate of the International Organization for Standardization (ISO), covers electrocardiographic monitoring equipment used in and outside the hospital environment, such as ambulances and air transport. This particular standard also applies to ECG telemetry systems used in the hospital environment.
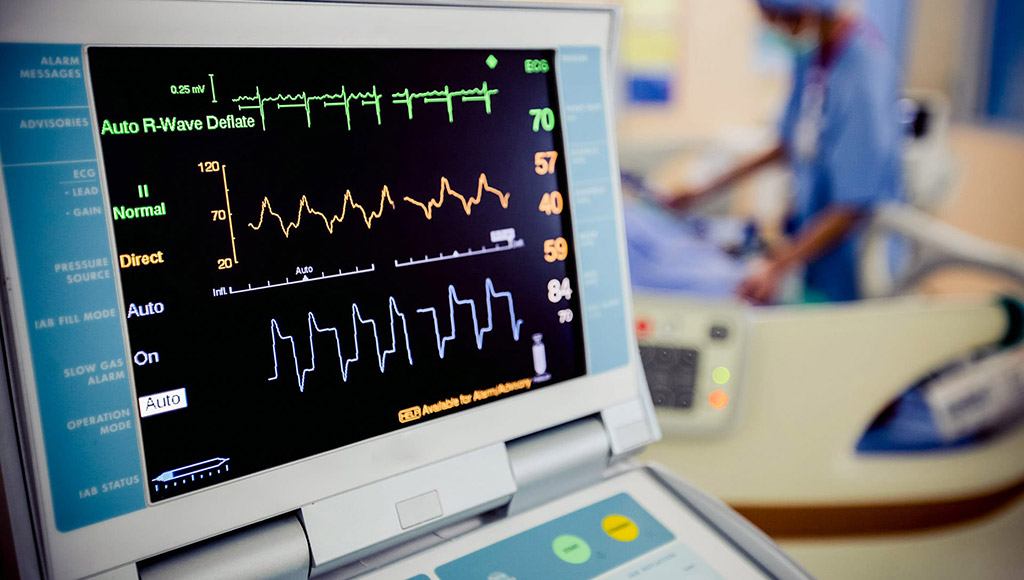
This standard has been published in our country by the Turkish Standards Institute with the following title: TS EN 60601-2-27 Electrical medical equipment - Part 2-27: Specific features for the basic safety and required performance of electrocardiography monitoring equipment.
This standard describes the criteria for electrocardiographic monitoring devices intended for use in uncontrolled and difficult environmental conditions, such as ambulances and air transport outside the hospital environment. In addition, if there are other standards for these usage environments, these criteria also apply. However, this standard does not apply to electrocardiographic monitoring devices designed for home use. Nevertheless, manufacturers should consider the relevant criteria of this standard in their production programs in accordance with their intended use.
Outpatient monitoring (holter), fetal heart rate monitoring, organ growth monitoring and other ECG recording devices are not covered by this standard.
IEC The following standards referred to during the implementation of the 60601-2-27 standard are indispensable: IEC 60601-1-2 (electromagnetic compatibility), IEC 60601-1-8 (general requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systems) , IEC 60601-2-2 (specific requirements for basic safety and basic performance of high-frequency surgical equipment and high-frequency surgical accessories), IEC 60601-2-25 (specific rules for basic safety and basic performance of electrocardiographs) and IEC 60601-2- 49 (specific requirements for the basic safety and basic performance of multifunction patient monitoring equipment)
Tests in accordance with IEC 60601-2-27 standard are also carried out by our organization within the scope of medical device tests.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.