

EUROLAB, with its state-of-the-art accredited laboratories and expert team, provides precise and fast testing services within the scope of IEC EN 60601-2-40 testing. IEC EN 60601-2-40 is a specific standard within the IEC 60601 series that deals with the safety and performance requirements of medical electrical equipment. In this case, the standard focuses on "Special requirements for the basic safety and basic performance of medical electrical equipment, Electromyographs and evoked response equipment".
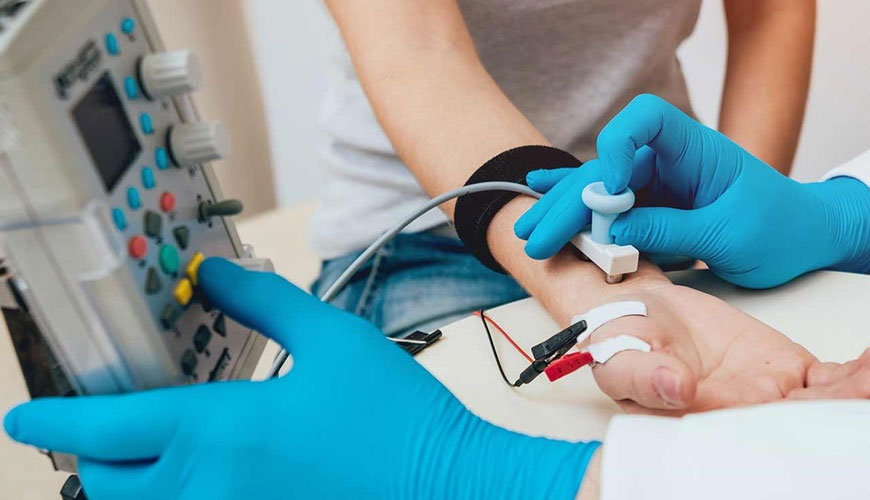
Electromyographs, commonly known as electromyography (EMG), are devices used to measure and record the electrical activity produced by skeletal muscles. Evoked response equipment is used to measure and record electrical responses in the nervous system stimulated by external stimuli such as visual, auditory, or sensory stimuli.
The IEC EN 60601-2-40 standard outlines the specific safety and performance requirements that such medical equipment must meet to ensure the safety of patients, users and operators, as well as the correct and reliable performance of the equipment. The standard covers various aspects such as electrical safety, mechanical safety, electromagnetic compatibility and usability.
Some of the key areas that the standard can address include:
It is important that manufacturers of electromyographs and evoked response equipment adhere to these standards to ensure their products meet the required safety and performance criteria. Additionally, healthcare facilities and professionals rely on these standards to ensure the safety and reliability of the medical equipment they use.
EUROLAB assists manufacturers with IEC EN 60601-2-40 test compliance. Our test experts, with their professional working mission and principles, provide you, our manufacturers and suppliers, the best service and controlled testing process in our laboratories. Thanks to these services, businesses receive more effective, high-performance and quality testing services and provide safe, fast and uninterrupted service to their customers.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.