
BS EN 8185 standard, which describes the general features and test methods of humidifiers and humidification systems used for medical purposes, was developed by the British Standards Institute (BSI). This standard, published by the Turkish Standards Institute (TSE) in our country, was later revoked and the ISO 80601-2-74 standard prepared by the International Standards Organization (ISO) was published with the following title: TS EN ISO 80601-2-74 Electrical Medical Equipment - Department 2-74: Specific requirements for the basic safety and required performance of the respiratory humidifier equipment.

The standard in question describes the features of a humidifier, called medical system, in combination with medical equipment and device, along with accessories, for basic safety and required performance.
This part of the international 80601 standard also applies to accessories intended for connection to a humidifier device. The features of these accessories can affect the basic safety or basic performance of the humidifier. For example, heated breathing tubes or medical equipment designed to control these heated breathing tubes. Heated breathing tubes and control devices are considered medical devices and are subject to the requirements of the IEC 60601‑1 standard.
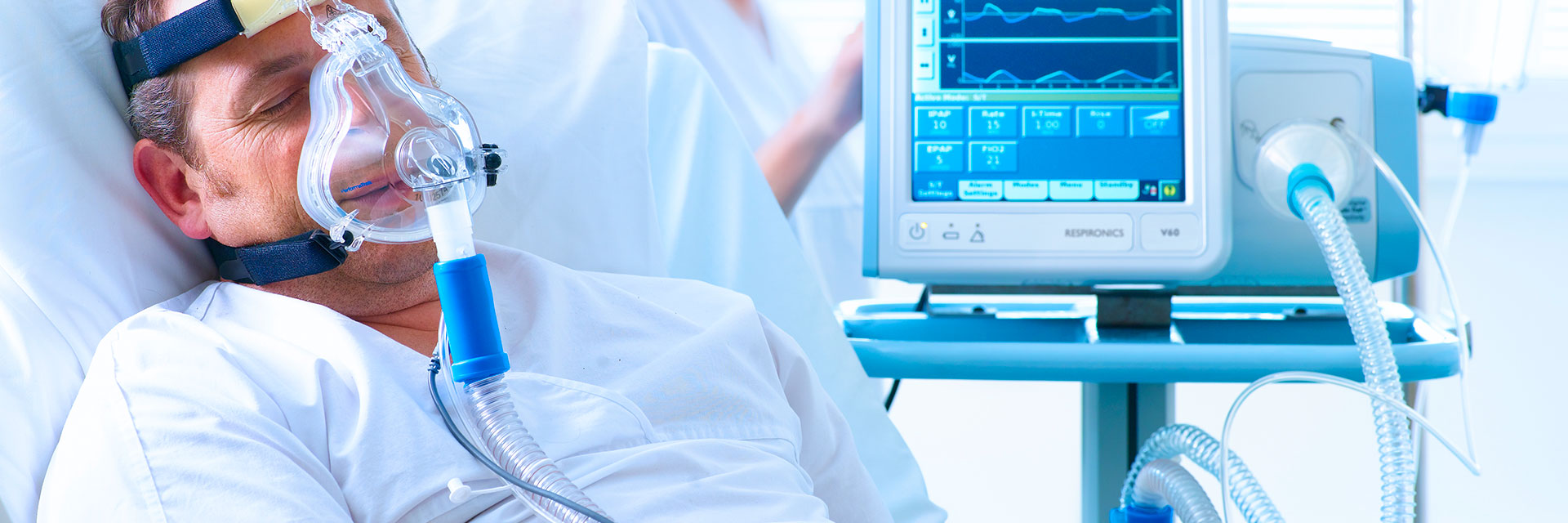
The standard also includes requirements for different medical uses of humidification, such as invasive ventilation, non-invasive ventilation, nasal high-flow therapy, obstructive sleep apnea therapy, and humidification therapy for tracheostomy patients.
Meanwhile, a humidifier can be integrated into other equipment. In this case, the requirements of other equipment are also considered valid for the humidifier.
The ISO 80601-2-74 standard also describes requirements for medical equipment used to increase the temperature and humidity level of the gas delivered to the patient.
Within the scope of breathing apparatus compatibility tests carried out by our organization EUROLAB, testing services in accordance with TS EN ISO 80601-2-74 standard are also provided.
To get an appointment, to get more detailed information or to request an evaluation, you can ask us to fill in our form and reach you.